Dexamethasone-17-acetate cas no:1177-87-3
Synonyms: Dex-Cortideltacetate;Dexa-Cortisyl;Fortecortin;Fortecortin (crystal suspension);Pregna-1,4-diene-3,20-dione,21-(acetyloxy)-9-fluoro-11,17-dihydroxy-16-methyl-, (11b,16a)-;Pregna-1,4-diene-3,20-dione,9-fluoro-11b,17,21-trihydroxy-16a-methyl-, 21-acetate (6CI,8CI);16a-Methyl-9a-fluoroprednisolone 21-acetate;Dalalone DP;Dalalone LA;Decadron LA;Decadronal;Dectancyl;Dexamethasone acetate;
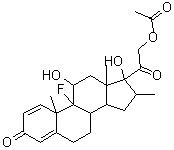
NameDexamethasone-17-acetate
CAS1177-87-3
SynonymsDex-Cortideltacetate;Dexa-Cortisyl;Fortecortin;Fortecortin (crystal suspension);Pregna-1,4-diene-3,20-dione,21-(acetyloxy)-9-fluoro-11,17-dihydroxy-16-methyl-, (11b,16a)-;Pregna-1,4-diene-3,20-dione,9-fluoro-11b,17,21-trihydroxy-16a-methyl-, 21-acetate (6CI,8CI);16a-Methyl-9a-fluoroprednisolone 21-acetate;Dalalone DP;Dalalone LA;Decadron LA;Decadronal;Dectancyl;Dexamethasone acetate;
EINECS(EC#)214-646-8
Molecular FormulaC24H31FO6
Molecular Weight434.5
AppearanceWhite or almost white crystalline powder
refractive index87 ° (C=1, Dioxane)
storage temp2-8°C
Hazard Xi:Irritant
Risk R43
Safety
Experimental teratogenic and reproductive effects. A steroid. When heated to decomposition it emits toxic fumes of F?.
Hazard Codes:  Xi
Xi
Risk Statements: 43
R43:May cause sensitization by skin contact ;
Safety Statements: 36/37
S36/37:Wear suitable protective clothing and gloves ;
WGK Germany: 3
RTECS: TU4050000
CHEMICAL IDENTIFICATION
- RTECS NUMBER :
- TU4050000
- CHEMICAL NAME :
- Pregna-1,4-diene-3,20-dione,
9-fluoro-11-beta,17,21-trihydroxy-16-alpha-methyl-,
acetate
- CAS REGISTRY NUMBER :
- 1177-87-3
- LAST UPDATED :
- 199701
- DATA ITEMS CITED :
- 12
- MOLECULAR FORMULA :
- C24-H31-F-O6
- MOLECULAR WEIGHT :
- 434.55
- WISWESSER LINE NOTATION :
- L E5 B666 OV AHTTT&J A1 BF CQ E1 FV1OV1 FQ G1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 1 mg/kg
- SEX/DURATION :
- female 15-19 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - post-implantation mortality (e.g. dead and/or
resorbed implants per total number of implants)
Reproductive - Fertility - abortion
- REFERENCE :
- FESTAS Fertility and Sterility. (American Fertility Soc., 608 13th Ave. S,
Birmingham, AL 35282) V.1- 1950- Volume(issue)/page/year: 22,735,1971
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 1200 ug/kg
- SEX/DURATION :
- female 12-13 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
- REFERENCE :
- TJADAB Teratology, The International Journal of Abnormal Development. (Alan
R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968-
Volume(issue)/page/year: 23,15,1981
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 1200 ug/kg
- SEX/DURATION :
- female 12-13 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Specific Developmental Abnormalities - body wall
- REFERENCE :
- DPTHDL Developmental Pharmacology and Therapeutics. (S. Karger Pub., Inc.,
79 Fifth Ave., New York, NY 10003) V.1- 1980- Volume(issue)/page/year:
4,89,1982
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 2400 ug/kg
- SEX/DURATION :
- female 12-13 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
- REFERENCE :
- DPTHDL Developmental Pharmacology and Therapeutics. (S. Karger Pub., Inc.,
79 Fifth Ave., New York, NY 10003) V.1- 1980- Volume(issue)/page/year:
4,89,1982
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 12800 ug/kg
- SEX/DURATION :
- female 9-12 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - post-implantation mortality (e.g. dead and/or
resorbed implants per total number of implants)
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
- REFERENCE :
- SEIJBO Senten Ijo. Congenital Anomalies. (Nippon Senten Ijo Gakkai, c/o
Kinki Daigaku Igakubu Kaibagaku Kyoshitsu, 380 Nishiyama, Sayama-cho,
Mirami-Kawachi-gun, Osaka-fu, Japan) V.1-26, 1960-86. For publisher
information, see CGANE7. Volume(issue)/page/year: 13,245,1973
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 12800 ug/kg
- SEX/DURATION :
- female 11-14 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
- REFERENCE :
- SEIJBO Senten Ijo. Congenital Anomalies. (Nippon Senten Ijo Gakkai, c/o
Kinki Daigaku Igakubu Kaibagaku Kyoshitsu, 380 Nishiyama, Sayama-cho,
Mirami-Kawachi-gun, Osaka-fu, Japan) V.1-26, 1960-86. For publisher
information, see CGANE7. Volume(issue)/page/year: 13,245,1973
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- DOSE :
- 128 mg/kg
- SEX/DURATION :
- female 11-14 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - post-implantation mortality (e.g. dead and/or
resorbed implants per total number of implants)
Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
- REFERENCE :
- SEIJBO Senten Ijo. Congenital Anomalies. (Nippon Senten Ijo Gakkai, c/o
Kinki Daigaku Igakubu Kaibagaku Kyoshitsu, 380 Nishiyama, Sayama-cho,
Mirami-Kawachi-gun, Osaka-fu, Japan) V.1-26, 1960-86. For publisher
information, see CGANE7. Volume(issue)/page/year: 13,245,1973
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Ocular
- DOSE :
- 1200 ug/kg
- SEX/DURATION :
- female 10-13 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
- REFERENCE :
- TJADAB Teratology, The International Journal of Abnormal Development. (Alan
R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968-
Volume(issue)/page/year: 16,175,1977
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Ocular
- DOSE :
- 6 mg/kg
- SEX/DURATION :
- female 10-13 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - urogenital system
- REFERENCE :
- TJADAB Teratology, The International Journal of Abnormal Development. (Alan
R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968-
Volume(issue)/page/year: 16,175,1977
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 6 mg/kg
- SEX/DURATION :
- female 24-26 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Newborn - biochemical and metabolic
- REFERENCE :
- BCPCA6 Biochemical Pharmacology. (Pergamon Press Inc., Maxwell House,
Fairview Park, Elmsford, NY 10523) V.1- 1958- Volume(issue)/page/year:
27,1007,1978
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 9 mg/kg
- SEX/DURATION :
- female 5-7 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - other measures of fertility
- REFERENCE :
- BIREBV Biology of Reproduction. (Soc. for the Study of Reproduction, 309 W.
Clark St., Champaign, IL 61820) V.1- 1969- Volume(issue)/page/year:
30,544,1984

 Xi
Xi