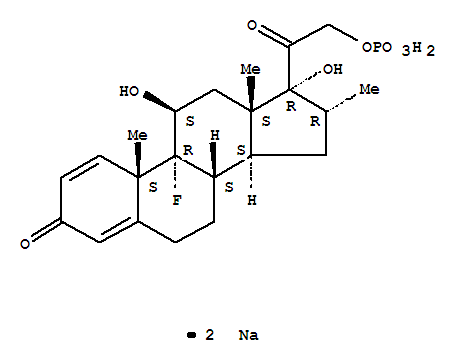
Pregna-1,4-diene-3,20-dione,9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, sodium salt (1:2), (11b,16a)- cas no:2392-39-4
Synonyms: Pregna-1,4-diene-3,20-dione,9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, disodium salt, (11b,16a)- (9CI);Dexagro;Dexamethasone 21-(disodium phosphate);Dexamethasone phosphate sodium salt;Dexamethazone sodium phosphate;Dezone;Disodium dexamethasone 21-phosphate;
NamePregna-1,4-diene-3,20-dione,9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, sodium salt (1:2), (11b,16a)-
CAS2392-39-4
SynonymsPregna-1,4-diene-3,20-dione,9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, disodium salt, (11b,16a)- (9CI);Dexagro;Dexamethasone 21-(disodium phosphate);Dexamethasone phosphate sodium salt;Dexamethazone sodium phosphate;Dezone;Disodium dexamethasone 21-phosphate;
EINECS(EC#)219-243-0
Molecular FormulaC22H30FO8P.2Na
Molecular Weight516.404624
AppearanceWhite to off white powder
storage temp2-8°C
Hazard Xn: Harmful;
Risk R22;R40;R63
Safety Poison by intravenous route. Moderately toxic by ingestion and intraperitoneal routes. Human systemic effects by intravenous route: peritonitis, central nervous system, and gastrointestinal changes. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of F?, POx, and Na2O.
CHEMICAL IDENTIFICATION
- RTECS NUMBER :
- TU4056000
- CHEMICAL NAME :
- Pregna-1,4-diene-3,20-dione,
9-fluoro-11-beta,17,21-trihydroxy-16-alpha-methyl-,
21-(dihydrogen phosphate) disodium salt
- CAS REGISTRY NUMBER :
- 2392-39-4
- LAST UPDATED :
- 199806
- DATA ITEMS CITED :
- 27
- MOLECULAR FORMULA :
- C22-H28-F-O8-P.2Na
- MOLECULAR WEIGHT :
- 516.45
- WISWESSER LINE NOTATION :
- L E5 B666 OV AHTTT&J A1 BF CQ E1 FV1OPWO FQ G1 &-NA-
2
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Human - infant
- DOSE/DURATION :
- 1500 ug/kg/3D-I
- TOXIC EFFECTS :
- Brain and Coverings - recordings from specific areas of CNS
- REFERENCE :
- LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823-
Volume(issue)/page/year: 1,632,1987
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Human - woman
- DOSE/DURATION :
- 320 mg/kg
- TOXIC EFFECTS :
- Gastrointestinal - other changes
- REFERENCE :
- LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823-
Volume(issue)/page/year: 1,1035,1986
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Human - child
- DOSE/DURATION :
- 1 mg/kg
- TOXIC EFFECTS :
- Gastrointestinal - peritonitis
- REFERENCE :
- AJEMEN American Journal of Emergency Medicine. (WB Saunders, Philadelphia,
PA) V.1- 1983- Volume(issue)/page/year: 10,268,1992
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Human - man
- DOSE/DURATION :
- 357 ug/kg
- TOXIC EFFECTS :
- Gastrointestinal - peritonitis
- REFERENCE :
- AJEMEN American Journal of Emergency Medicine. (WB Saunders, Philadelphia,
PA) V.1- 1983- Volume(issue)/page/year: 10,268,1992
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Oral
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 1800 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- ATSUDG Archives of Toxicology, Supplement. (Springer-Verlag New York, Inc.,
Service Center, 44 Hartz Way, Secaucus, NJ 07094) No.1- 1978-
Volume(issue)/page/year: 7,90,1984
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Intraperitoneal
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 550 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- ATSUDG Archives of Toxicology, Supplement. (Springer-Verlag New York, Inc.,
Service Center, 44 Hartz Way, Secaucus, NJ 07094) No.1- 1978-
Volume(issue)/page/year: 7,90,1984
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Intravenous
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 932 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo,
Japan) Volume(issue)/page/year: -,785,1995
** REPRODUCTIVE DATA **
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 7500 ug/kg
- SEX/DURATION :
- female 6-15 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetal death
- REFERENCE :
- BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica,
Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year:
119,391,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 1 mg/kg
- SEX/DURATION :
- female 6-15 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
- REFERENCE :
- BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica,
Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year:
119,391,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intraperitoneal
- DOSE :
- 400 ug/kg
- SEX/DURATION :
- female 19-20 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight
gain)
- REFERENCE :
- PEDIAU Pediatrics. (American Academy of Pediatrics, P.O. Box 1034,
Evanston, IL 60204) V.1- 1948- Volume(issue)/page/year: 65,287,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 10 mg/kg
- SEX/DURATION :
- female 6-15 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Effects on Embryo or Fetus - fetal death
- REFERENCE :
- BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica,
Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year:
119,391,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 2 mg/kg
- SEX/DURATION :
- female 6-15 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
- REFERENCE :
- BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica,
Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year:
119,391,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Subcutaneous
- DOSE :
- 12800 ug/kg
- SEX/DURATION :
- female 11-14 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - post-implantation mortality (e.g. dead and/or
resorbed implants per total number of implants)
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
- REFERENCE :
- SEIJBO Senten Ijo. Congenital Anomalies. (Nippon Senten Ijo Gakkai, c/o
Kinki Daigaku Igakubu Kaibagaku Kyoshitsu, 380 Nishiyama, Sayama-cho,
Mirami-Kawachi-gun, Osaka-fu, Japan) V.1-26, 1960-86. For publisher
information, see CGANE7. Volume(issue)/page/year: 13,245,1973
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 130 ug/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetal death
- REFERENCE :
- BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica,
Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year:
119,391,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 650 ug/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
- REFERENCE :
- BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica,
Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year:
119,391,1980
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intramuscular
- DOSE :
- 9 mg/kg
- SEX/DURATION :
- female 21-26 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Maternal Effects - parturition
Reproductive - Effects on Embryo or Fetus - fetal death
- REFERENCE :
- JOENAK Journal of Endocrinology. (Biochemical Soc. Book Depot, POB 32,
Commerce Way, Colchester, Essex CO2 8HP, UK) V.1- 1939-
Volume(issue)/page/year: 64,363,1975
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intramuscular
- DOSE :
- 300 ug/kg
- SEX/DURATION :
- female 25-27 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - gastrointestinal
system
- REFERENCE :
- JSGRA2 Journal of Surgical Research. (Academic Press, Inc., 1 E. First St.,
Duluth, MN 55802) V.1- 1961- Volume(issue)/page/year: 57,274,1994
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Parenteral
- DOSE :
- 8 mg/kg
- SEX/DURATION :
- female 11-14 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Specific Developmental Abnormalities - Central Nervous System
Reproductive - Specific Developmental Abnormalities - craniofacial
(including nose and tongue)
Reproductive - Specific Developmental Abnormalities - body wall
- REFERENCE :
- ANIFAC Annales de Chirurgie Infantile. (Paris, France) V.1- 1960(?)-
Volume(issue)/page/year: 3,73,1962
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Parenteral
- DOSE :
- 8 mg/kg
- SEX/DURATION :
- female 11-14 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Effects on Embryo or Fetus - fetal death
- REFERENCE :
- ANIFAC Annales de Chirurgie Infantile. (Paris, France) V.1- 1960(?)-
Volume(issue)/page/year: 3,73,1962
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Parenteral
- DOSE :
- 4 mg/kg
- SEX/DURATION :
- female 11 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Fertility - post-implantation mortality (e.g. dead and/or
resorbed implants per total number of implants)
Reproductive - Fertility - abortion
- REFERENCE :
- ANIFAC Annales de Chirurgie Infantile. (Paris, France) V.1- 1960(?)-
Volume(issue)/page/year: 3,73,1962
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intramuscular
- DOSE :
- 145 ug/kg
- SEX/DURATION :
- female 20 week(s) after conception
- TOXIC EFFECTS :
- Reproductive - Maternal Effects - parturition
- REFERENCE :
- AAAHAN Australian Journal of Experimental Agriculture and Animal Husbandry.
(Commonwealth Scientific and Industrial Research Organization, 314 Albert
St., E. Melbourne, Vic. 3002, Australia) V.1- 1961-
Volume(issue)/page/year: 16,462,1976
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intramuscular
- DOSE :
- 2739 ug/kg
- SEX/DURATION :
- female 21 day(s) pre-mating
- TOXIC EFFECTS :
- Reproductive - Maternal Effects - other effects
- REFERENCE :
- JRFSAR Journal of Reproduction and Fertility, Supplement. (Biochemical Soc.
Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) No.1-
1966- Volume(issue)/page/year: 32,247,1982
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Intramuscular
- DOSE :
- 1304 ug/kg
- SEX/DURATION :
- female 10 day(s) pre-mating
- TOXIC EFFECTS :
- Reproductive - Fertility - other measures of fertility
- REFERENCE :
- JRFSAR Journal of Reproduction and Fertility, Supplement. (Biochemical Soc.
Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) No.1-
1966- Volume(issue)/page/year: 32,247,1982
*** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA ***
NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA :
NOHS - National Occupational Hazard Survey (1974)
NOHS Hazard Code - 82148
No. of Facilities: 67 (estimated)
No. of Industries: 2
No. of Occupations: 1
No. of Employees: 680 (estimated)
NOES - National Occupational Exposure Survey (1983)
NOES Hazard Code - 82148
No. of Facilities: 363 (estimated)
No. of Industries: 2
No. of Occupations: 7
No. of Employees: 16943 (estimated)
No. of Female Employees: 14180 (estimated)