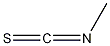Hazard T:Toxic;N:Dangerousfortheenvironment;
Risk R23/25;R34;R43;R50/53
Safety
A poison by ingestion, skin contact, and subcutaneous routes. Very irritating to skin, eyes, and mucous membranes. Human systemic effects by ingestion: convulsions, change in motor activity, coma. An agricultural chemical and pesticide. Flammable when exposed to heat or flame; can react vigorously with oxidizing materials. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also THIOCYANATES.
Hazard Codes:
T: Toxic .gif)
N: Dangerous for the environment .gif)
Risk Statements about Isothiocyanatomethane (556-61-6):
R23/25 Toxic by inhalation and if swallowed.
R34 Causes burns.
R43 May cause sensitization by skin contact.
R50/53 Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements about Isothiocyanatomethane (556-61-6):
S36/37 Wear suitable protective clothing and gloves.
S38 In case of insufficient ventilation, wear suitable respiratory equipment.
S45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible).
S60 This material and its container must be disposed of as hazardous waste.
S61 Avoid release to the environment. Refer to special instructions/safety data sheets.
Attention:
1. Storage: Keep away from sources of ignition. Do not store in direct sunlight. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
2. Handling: Use spark-proof tools and explosion proof equipment. Loosen closure cautiously before opening. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
3. Reactivity Profile: Isocyanates and thioisocyanates, such as Methyl isothiocyanate, are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Polyurethanes are formed by the condensation reaction of diisocyanates with, for example, ethyl glycol.