Hazard T:Toxic
Risk R23;R34
Safety
Hazard Codes of Sulfur dioxide (CAS NO.7446-09-5):? T
T
Risk Statements: 23-34?
R23: Toxic by inhalation.?
R34: Causes burns.
Safety Statements: 9-26-36/37/39-45?
S9: Keep container in a well-ventilated place.?
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.?
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.?
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 2037 2.3
WGK Germany: 1
RTECS: WS4550000
F: 4.5-31
HazardClass: 2.3
A poison gas. Experimental reproductive effects. Human mutation data reported. Human systemic effects by inhalation: pulmonary vascular resistance, respiratory depression, and other pulmonary changes. Questionable carcinogen with experimental tumorigenic and teratogenic data. It chiefly affects the upper respiratory tract and the bronchi. It may cause edema of the lungs or glottis, and can produce respiratory paralysis. A corrosive irritant to eyes, skin, and mucous membranes. This material is so irritating that it provides its own warning of toxic concentration. Levels of 400–500?ppm are immediately dangerous to life. Its toxicity is comparable to that of hydrogen chloride. However, less than fatal concentration can be borne for fair periods of time with no apparent permanent damage. It is a common air contaminant.
A nonflammable gas. It reacts violently with acrolein, Al, CsHC2, Cs2O, chlorates, ClF3, Cr, FeO, F2, Mn, KHC2, KClO3, Rb2C2, Na, Na2C2, SnO, diaminolithiumacetylene carbide. Will react with water or steam to produce toxic and corrosive fumes. Incompatible with halogens or interhalogens, lithium nitrate, metal acetylides, metal oxides, metals, polymeric tubing, potassium chlorate, sodium hydride.

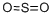
 T
T