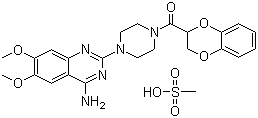
Methanone,[4-(4-amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](2,3-dihydro-1,4-benzodioxin-2-yl)-,methanesulfonate (1:1) cas no:77883-43-3
Synonyms: Normothen;Supressin;UK 33274-27;doxazolazine mesylate;Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(2,3-dihydro-1,4-benzodioxin-2-yl)carbonyl]-,monomethanesulfonate (9CI);Alfadil;Cardenalin;Cardran;Cardular;Cardura;Cardura (pharmaceutical);Carduran;Diblocin;Doxazosin mesylate;Doxazosin monomethanesulfonate;
NameMethanone,[4-(4-amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](2,3-dihydro-1,4-benzodioxin-2-yl)-,methanesulfonate (1:1)
CAS77883-43-3
SynonymsNormothen;Supressin;UK 33274-27;doxazolazine mesylate;Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(2,3-dihydro-1,4-benzodioxin-2-yl)carbonyl]-,monomethanesulfonate (9CI);Alfadil;Cardenalin;Cardran;Cardular;Cardura;Cardura (pharmaceutical);Carduran;Diblocin;Doxazosin mesylate;Doxazosin monomethanesulfonate;
Molecular FormulaC24H29N5O8S
Molecular Weight547.58076
Appearancewhite to off-white crystalline powder
storage tempDesiccate at RT
Hazard UN
NO.
Risk R36/37/38
Safety Hazard Codes:Xi
Risk Statements:36/37/38
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:26-36/37
26:In case of contact with eyes, rinse immediately with plenty
of water and seek medical advice
36/37:Wear suitable protective clothing and gloves
WGK Germany:2
Hazardous Substances Data:77883-43-3(Hazardous Substances Data)
CHEMICAL IDENTIFICATION
- RTECS NUMBER :
- TK8044000
- CHEMICAL NAME :
- Piperazine,
1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-((2,3-dihy
dro-1,4-benzodioxin-2 -yl) carbonyl)-,
monomethanesulfonate
- CAS REGISTRY NUMBER :
- 77883-43-3
- LAST UPDATED :
- 199109
- DATA ITEMS CITED :
- 11
- MOLECULAR FORMULA :
- C23-H25-N5-O5.C-H4-O3-S
- MOLECULAR WEIGHT :
- 547.64
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Oral
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- >5 gm/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo,
Japan) Volume(issue)/page/year: -,712,1990
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Intraperitoneal
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- 380 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo,
Japan) Volume(issue)/page/year: -,712,1990
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - rat
- DOSE/DURATION :
- >5 gm/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo,
Japan) Volume(issue)/page/year: -,712,1990
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Oral
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 2935 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo,
Japan) Volume(issue)/page/year: -,712,1990
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Intraperitoneal
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 247 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo,
Japan) Volume(issue)/page/year: -,712,1990
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Subcutaneous
- SPECIES OBSERVED :
- Rodent - mouse
- DOSE/DURATION :
- 4087 mg/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo,
Japan) Volume(issue)/page/year: -,712,1990
- TYPE OF TEST :
- LD50 - Lethal dose, 50 percent kill
- ROUTE OF EXPOSURE :
- Oral
- SPECIES OBSERVED :
- Mammal - dog
- DOSE/DURATION :
- >1 gm/kg
- TOXIC EFFECTS :
- Details of toxic effects not reported other than lethal dose value
- REFERENCE :
- NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo,
Japan) Volume(issue)/page/year: -,712,1990
** REPRODUCTIVE DATA **
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 1320 mg/kg
- SEX/DURATION :
- female 7-17 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetal death
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,29,1990
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 520 mg/kg
- SEX/DURATION :
- female 17-21 day(s) after conception
lactating female 21 day(s) post-birth
- TOXIC EFFECTS :
- Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4
per # born alive)
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,29,1990
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 1300 mg/kg
- SEX/DURATION :
- female 17-21 day(s) after conception
lactating female 21 day(s) post-birth
- TOXIC EFFECTS :
- Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight
gain)
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,29,1990
- TYPE OF TEST :
- TDLo - Lowest published toxic dose
- ROUTE OF EXPOSURE :
- Oral
- DOSE :
- 1300 mg/kg
- SEX/DURATION :
- female 6-18 day(s) after conception
- TOXIC EFFECTS :
- Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death,
e.g., stunted fetus)
Reproductive - Effects on Embryo or Fetus - fetal death
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight
gain)
- REFERENCE :
- OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180,
Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,29,1990